Electrodes 🔋 🔋 ⚡️ ⚡️
As it is from my interest to know and learn about Chemistry and science (for more reference, check my Chemistry: A Truly Blue Sky blog), I visited Professor Jang Wook Choi from Seoul National University, as he tutored me about the basics of batteries and electrodes.
 First, I learned that in a redox reaction, between two metals (for example, zinc and copper are placed like the image shown), when the zinc metal gives off electrons, zinc is currently being oxidized because it is giving its electrons, while copper is being oxidized, because it is receiving electrons from the zinc metal.
First, I learned that in a redox reaction, between two metals (for example, zinc and copper are placed like the image shown), when the zinc metal gives off electrons, zinc is currently being oxidized because it is giving its electrons, while copper is being oxidized, because it is receiving electrons from the zinc metal.
 Now, through this knowledge, we can compare what is the structural difference between chargeable and dischargeable batteries. Another fact I learned was intercalation, which is the reversible inclusion or insertion of a molecule (or ion) into materials with layered structures.
Now, through this knowledge, we can compare what is the structural difference between chargeable and dischargeable batteries. Another fact I learned was intercalation, which is the reversible inclusion or insertion of a molecule (or ion) into materials with layered structures.
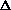 G = G prod - G react
G = G prod - G react
As this is sort of my introductory blog, it is my role to summarize what I have learned from him.
 First, I learned that in a redox reaction, between two metals (for example, zinc and copper are placed like the image shown), when the zinc metal gives off electrons, zinc is currently being oxidized because it is giving its electrons, while copper is being oxidized, because it is receiving electrons from the zinc metal.
First, I learned that in a redox reaction, between two metals (for example, zinc and copper are placed like the image shown), when the zinc metal gives off electrons, zinc is currently being oxidized because it is giving its electrons, while copper is being oxidized, because it is receiving electrons from the zinc metal.
As the image demonstrates, the one who gives off is also called an anode, and the one who receives is called cathode. The chemical reaction that happens in this instance shows that there are two independent half-reactions.
Well, as the diagram shows, there are some inquiries that may seem to question oneself: Can the reverse process happen, in which the copper is the anode and the zinc is the cathode? And the answer is no.
Another question is what makes this process to happen itself, and the answer is that the voltmeter in the image allows the electrons from the zinc to be transfers to the copper.
Anode and cathodes are types of electrodes, and when electrodes' ions are dissolved in the solvent, which in this case is water, it is called an electrolyte. For example, lithium-ion batteries have lithium dissolved in the solvent.
Volt = Joule/coulomb
 Now, through this knowledge, we can compare what is the structural difference between chargeable and dischargeable batteries. Another fact I learned was intercalation, which is the reversible inclusion or insertion of a molecule (or ion) into materials with layered structures.
Now, through this knowledge, we can compare what is the structural difference between chargeable and dischargeable batteries. Another fact I learned was intercalation, which is the reversible inclusion or insertion of a molecule (or ion) into materials with layered structures.
Like the diagram is shown left, (the right diagram is the chargeable battery 🔋 ) while the dischargeable battery only lets the electrons and lithium through above pathway and the separator respectively to only one direction, while in the chargeable battery, once it is plugged in for charging, the electrons go against their current flow as well as the lithiums that travel back towards their anode for charging.
Another good equation to consider is
Comments
Post a Comment